The healthcare environment has changed almost beyond recognition in the past decade and this has been well documented. One of the key upshots of the turbulence caused by healthcare reforms, patent cliffs, drying product pipelines, fiscal restraint and a host of other factors, is that many pharmaceutical companies have shifted their attention to specialist care and rare disease products. So it’s easy to see why companies want to invest in this area. But what is the best way to bringing such products to market?
Given the often very small patient numbers and specialist treatment centers associated with niche specialty care and orphan drugs, more tailored and nimble commercial models are required to get them into the hands of the patients who need them. A potential solution is the creation of a ‘supranational’ business unit, which sits above country borders, potentially at a regional level or at a ‘cluster level’ (serving a certain number of Centers of Excellence). This business unit can connect rare disease Centers of Excellence, KOLs and patients across different countries, managing them all from a single, central resource. It also provides organizations with visibility over people and processes involved in one disease area, with central monitoring of customer facing roles.
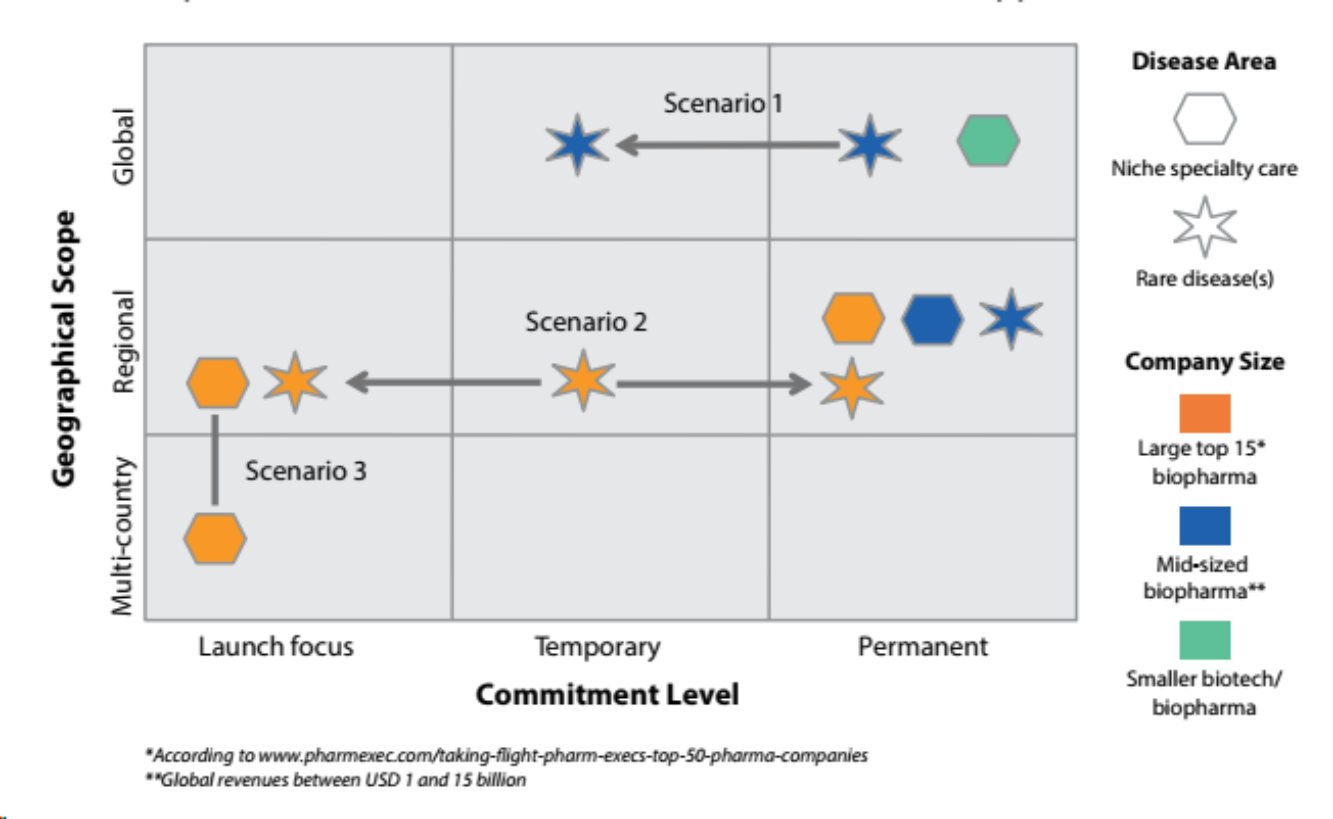
Several such models have been successfully developed and implemented in recent years, and many biopharmaceutical companies have implemented new organizational structures separate from their other organizations typically set-up in affiliate structures (see figure).
So the good news is that many successful organizational models have been implemented already. Some are set in stone and are here to stay; others have been set-up temporarily, often in transitionary periods such as for a successful launch.