Globally pharmaceutical companies are under immense pressure, with existing lobally pharmaceutical business models under threat. The growth is expected to taper off in the US and other developed countries, while emerging economies are expected to drive a large part of the future growth.
Declining productivity, relatively dry pipeline for new drugs, increasing penetration of generics and margin pressures have been leading to lower profitability for global pharmaceutical companies. This trend is expected to further intensify going forward into the future. This has forced companies to continuously adapt their cost structures, as exemplified by major multibillion cost cutting/restructuring projects in all major pharmaceutical companies.
The pharmaceutical industry is fundamentally re-evaluating the makeup of its value chain, differentiating clearly between core capabilities and those that could be potentially outsourced. Within R&D, particularly pre-clinical and discovery seem to be representing potential outsourcing opportunities, also driven by huge cost differences. In the future, the pharmaceutical industry will be forced to capture the increasing benefits from emerging countries, particularly given the longterm benefit from matching better its global work force footprint to the future geographic distribution of revenues.
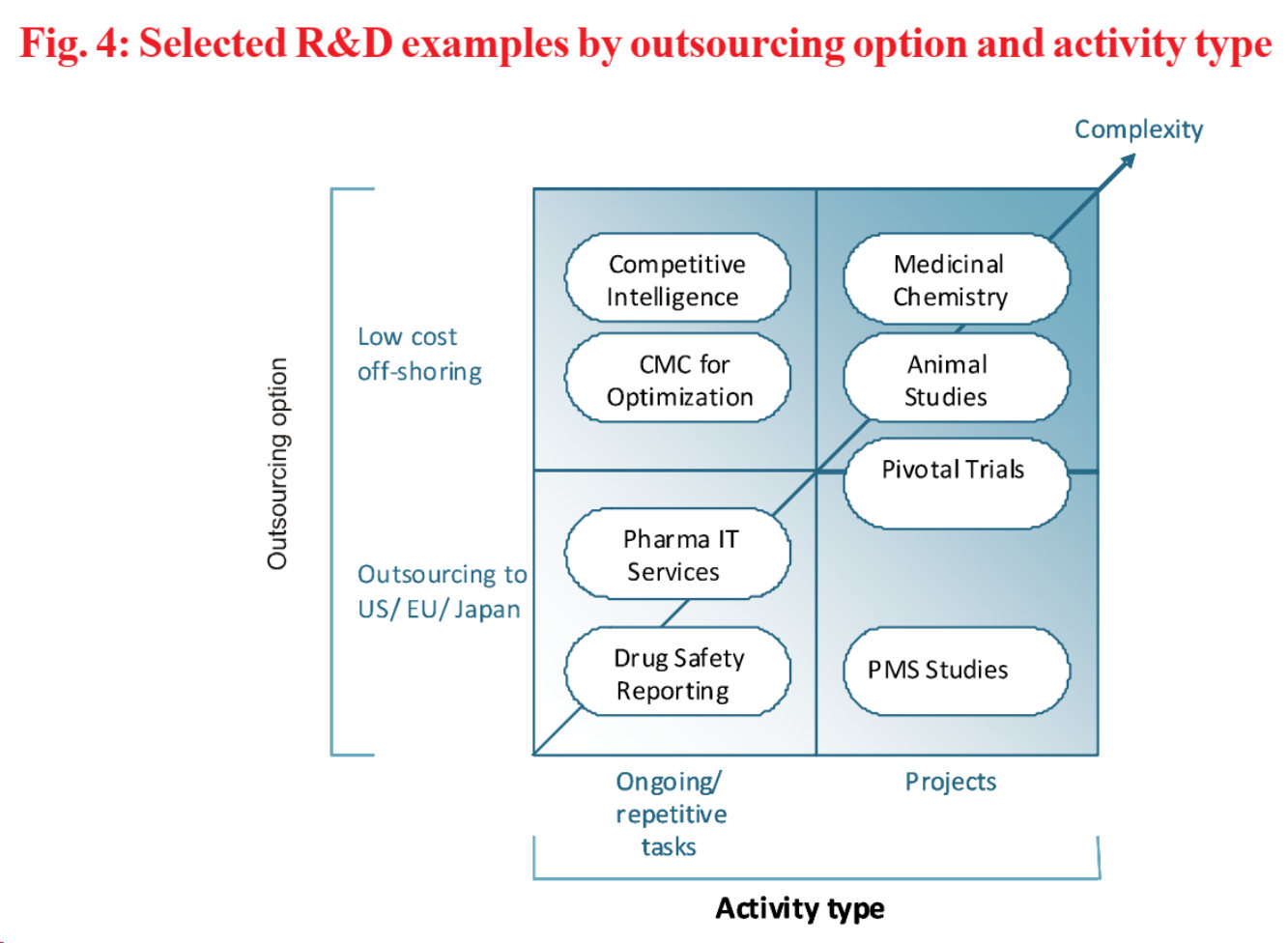
Pharmaceutical companies need to decide on their geographic footprint by assessing the various locations rigorously against a predefined set of key success factors. In addition, we suggest differentiating outsourcing decisions by activity type (differentiating ongoing/ repetitive tasks from projects) and outsourcing option (off-shoring leading to a strategic cost advantage vs. outsourcing within US/ EU/ Japan). Near and off-shoring seems to be equally driven by unit cost advantages, e.g. animal studies, as well as critical resource access, e.g. patients meeting clinical trial inclusion criteria, experienced medicinal chemists.
Given the advantages of focus, cost and speed, the question is no longer about whether to outsource, but rather of finding the right partners. Overall, clinical development, discovery and non-clinical services costs account for 85% of R&D budget, which can be reduced by using CROs. In addition to cost advantages, multinational pharmaceutical companies benefit from staying closer to schedule and their ability to expand speed and capacity of their R&D operations while maintaining high levels of quality, resulting in a much required boost of R&D productivity.
It is a foregone conclusion that pharmaceutical and biotech companies need to relook at their business models, if they have to successfully compete in the new environment. Contract manufacturing was just the tip of the iceberg. If companies have to be really successful and optimize their operations for better business results, they need to revamp their R&D process and capture the opportunity presented by emerging economies.
Price realizations that the pharma companies have got used to may be a thing of the past, especially with focus on reducing final cost of dose by payers and governments, even in the developed world. Off-shoring contract research (and manufacturing) services are therefore an opportunity to not just save costs tactically for the short term, but also a strategic move to improve productivity and develop further capabilities, while also moving closer to the future healthcare customers in developing markets.