To be more than a reaction to the Internet, individual biopharma initiatives to influence the patient/physician flow need to be combined into an overall digital marketing programme. These programmes will depend on the life cycle stage of the product, and differ for pre-launch, launch, Rx-to-OTC, and patent expiry. Even as they change over time, all programmes need a strategic consistency that unites content, presentation and interaction in a compelling fashion to achieve marketing objectives.
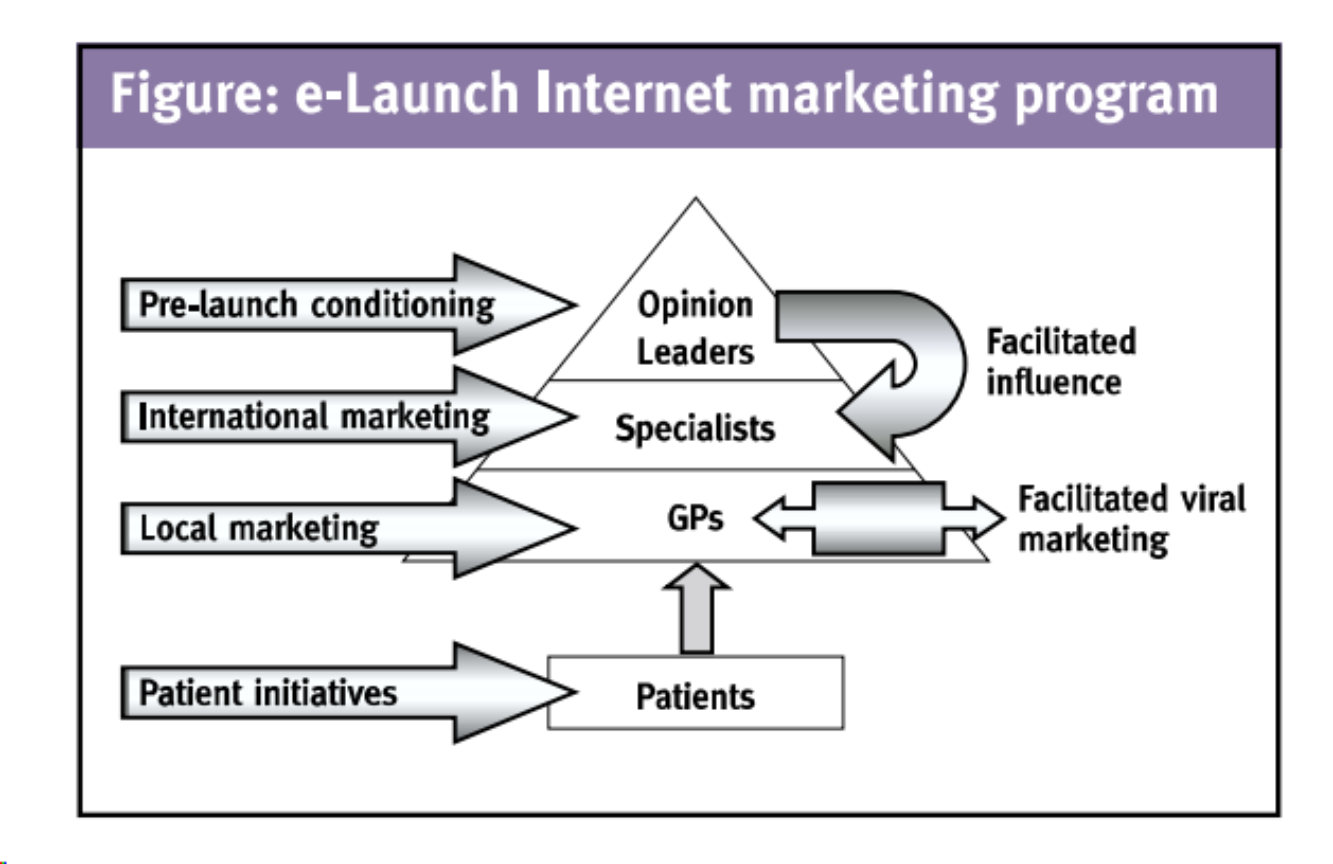
Let’s focus on the most important step, the launch of a pharmaceutical product. Similar to traditional launch programmes, where companies have adopted sophisticated approaches to target physicians and patients, they will have to adopt an orchestrated and carefully timed digital launch programme. It might start with prelaunch activities targeted at opinion leaders, and making them familiar with the new product. These opinion leaders can subsequently influence the relevant early adopters, who are interested to follow opinion leader discussions. The majority of prescribing physicians, specialists and/or general practitioners, are targeted through suppliers of emarketing and sales services, such as e-detailing, and online Phase IV trials. Marketers can also exploit the Internet to facilitate viral marketing among those physicians. Depending on the product and local regulation, marketers will target patients with general awareness programmes, product branding initiatives, and compliance/disease management initiatives.